In the rapidly evolving world of energy storage, two types of batteries have been making headlines: Sodium-ion batteries (SIBs) and Lithium-iron-phosphate batteries (LFP batteries). Both sodium battery technology and lithium battery technology are promising technologies, but they have distinct characteristics that make them suitable for different applications. In this article, we will explore what sodium-ion and lithium-iron-phosphate batteries are, and then compare their differences based on recent research findings.
What are Sodium-ion Batteries (SIBs)?
Sodium-ion batteries (SIBs) are a type of rechargeable battery that uses sodium ions (Na+) as the charge carriers. Sodium is abundant and inexpensive, making SIBs a new battery technology to replace lithium.
SIBs typically use hard carbon as the anode material, which is different from the graphite commonly used in LIBs. The cathode materials can vary, but they are often designed to accommodate the larger size of sodium ions compared to lithium ions.

What are LFP Batteries (Lithium-iron-phosphate Battery)?
Lithium-iron-phosphate batteries (LFP batteries) are a subtype of lithium-ion battery storage that use lithium iron phosphate (LiFePO4) as the cathode material.
Lithium LiFePO4 batteries are known for their thermal stability, long cycle life, and safety.
They are widely used in electric vehicles (EVs), renewable energy storage, and other applications where safety and longevity are critical.

Sodium-ion Battery VS Lithium Ion Battery

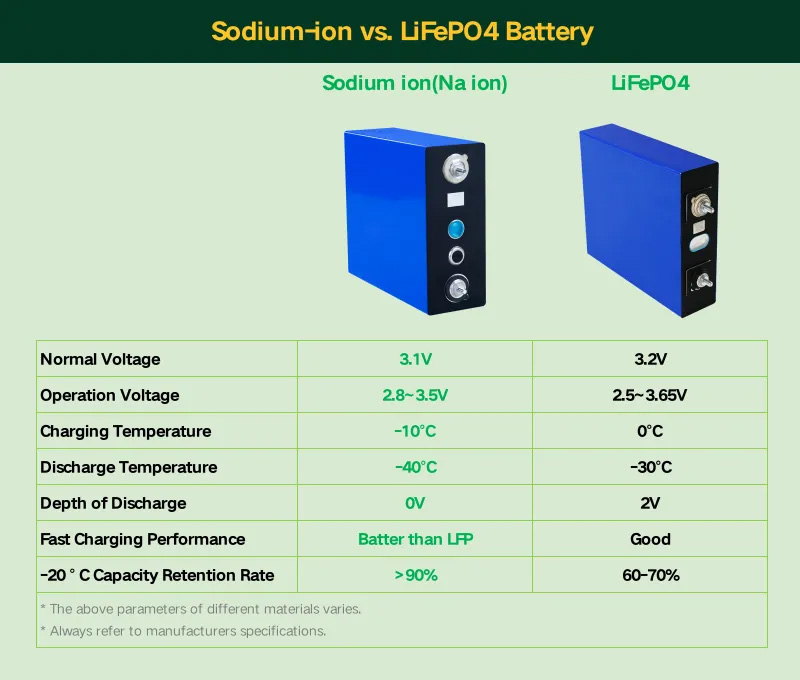
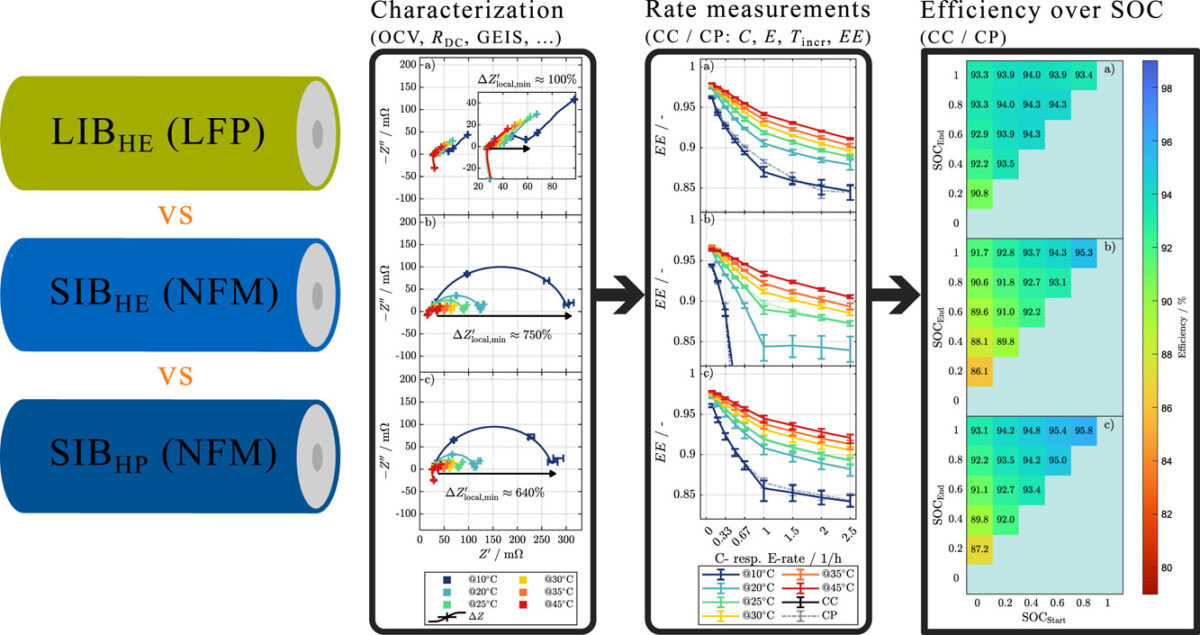
Image: Technical University of Munich (TUM), Journal of Power Sources, CC BY 4.0
|
Comparison Criteria |
Sodium-ion Battery | Lithium-iron-phosphate Battery |
| Electrical Performance | - More sensitive to state of charge (SOC) and temperature. - Pulse resistance and impedance increase significantly at low SOC (<30%) but decrease at high SOC. |
- Minimal dependence on SOC and temperature. - Stable resistance and impedance across varying SOC and temperature. |
| Anode Material | Uses hard carbon as the anode material, suitable for sodium ion intercalation and deintercalation. | Uses graphite as the anode material, suitable for lithium ion intercalation and deintercalation. |
| Efficiency and Energy Loss | - Efficiency highly dependent on SOC. - Energy loss significantly reduced when cycled between 50%-100% SOC. |
- Efficiency less dependent on SOC. - Maintains consistent efficiency across a wide range of SOC. |
| Cost and Material Abundance |
- Sodium is abundant and low-cost, offering potential cost advantages. - Technology and manufacturing processes are still developing, which may offset short-term cost benefits. |
- Lithium is relatively scarce and more expensive. - Mature manufacturing processes and established supply chain make it cost-competitive in the short term. |
| Applications | - Suitable for cost-sensitive applications, such as grid energy storage. - Ideal for applications where weight and size are less critical. |
- Suitable for applications requiring high safety and stability, such as electric vehicles and solar energy storage. - Ideal for scenarios requiring long cycle life and high reliability. |
| Temperature Sensitivity | - Performance fluctuates more under low or high temperatures. - Temperature changes significantly affect resistance and impedance. |
- Stable performance across a wide temperature range. - Temperature changes have minimal impact on performance. |
| Energy Density | - Lower energy density, suitable for applications where energy density is not a critical factor. | - Higher energy density, suitable for applications requiring high energy density, such as electric vehicles. |
| Safety | - Good safety, but hard carbon anode may cause hysteresis. | - Excellent safety, high thermal stability, and low risk of thermal runaway. |
| Research and Development | - Technology is still under development, with research focused on optimizing anode and cathode materials to improve performance. |
- Mature technology, with research focused on further improving energy density and reducing costs. |
Summary:
- ⭐ Sodium-ion batteries (SIBs) offer advantages in cost and material abundance, but are more sensitive to temperature and SOC, making them suitable for cost-sensitive applications with less stringent performance requirements.
- ⭐ LiFePO4 Solar Batteries excel in stability, safety, and efficiency, making them ideal for applications requiring high performance, safety, and long lifespan.
This table provides a clear and intuitive comparison of the two battery technologies, helping decision-makers choose the most suitable option based on specific needs.
Conclusion
Both Sodium-ion and lithium ion phosphate batteries have their unique advantages and challenges. Sodium batteries offer the potential for lower costs due to the abundance of sodium, but they are more sensitive to changes in SOC and temperature, which can affect their efficiency. On the other hand, LiFePO4 lithium batteries provide stable performance, long cycle life, and high safety, making them ideal for a wide range of applications, especially where reliability is crucial.
As research continues, we can expect further advancements in both technologies, potentially leading to new applications and improved performance. For now, the choice between Sodium-ion and lithium phosphate batteries will depend on the specific requirements of the application, including cost, performance, and safety considerations.
By understanding the differences between these two types of batteries, companies can make more informed decisions about which technology best suits their needs, whether they are producing batteries for electric vehicles, renewable energy storage, or other applications.
▲ More battery knowledge, please click here: https://www.youth-power.net/faqs/. Any lithium LiFePO4 battery inquiry or questions, please feel free to contact us at sales@youth-power.net.
